Within the fast-growing genomics universe, one of the more compelling themes is the evolution of gene therapies – a theme the ARK Genomic Revolution Multi-Sector Fund (CBOE: ARKG) is levered to.
Relative to passive genomics exchange traded funds, the actively managed ARKG can more rapidly adjust gene therapy exposure. For investors, that’s an exciting prospects because gene therapies are the epicenters of improving patient outcomes and, in some cases, cures.
One way that ARKG fits into this equation is that more gene therapy research is indicating long-read sequencing could be a pivotal long-term solution.
“Recently, scientists at the Franics Crick Institute used CRISPR Cas9 to edit embryos and found off-target edits, or unintended changes to the genome, in 16% of them. These off-target edits suggest that more detailed sequencing techniques, perhaps long read sequencing, will be necessary to ensure the safety of gene-editing,” according to a recent note from ARK Investment Management.
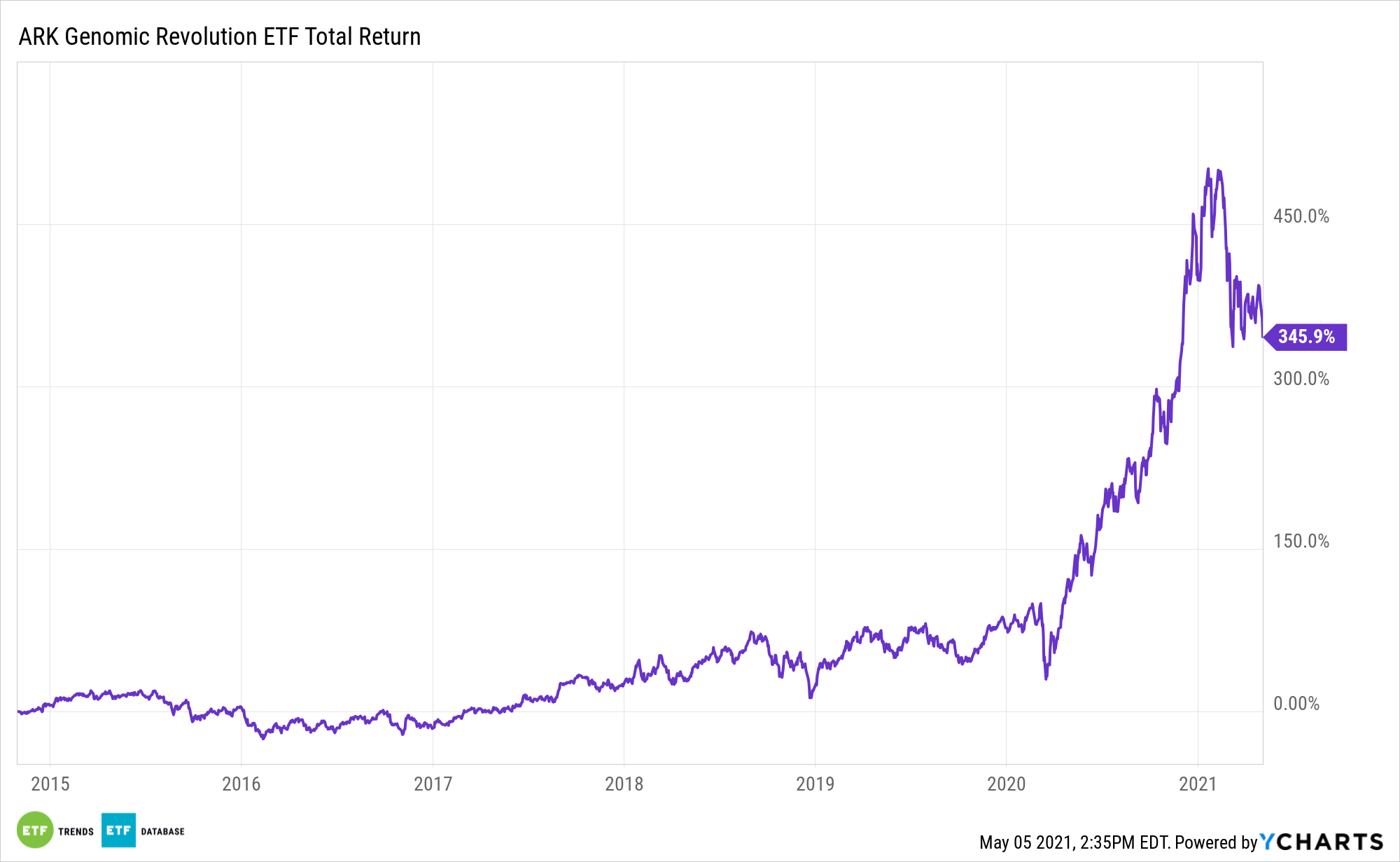
ARKG: Dodging Genomics Pitfalls
Among healthcare ETFs, ARKG is one of the more dominant names when it comes to adequate exposure to long-read sequencing. For investors, that’s an important attribute because that technology can help biotechnology companies avoid some of the pitfalls of gene therapy development and research.
“Adding more trepidation to the debate, several companies including Blue Bird Bio have reported serious adverse events (SAE) from their gene therapy trials. Interpreting whether the SAEs are a function of gene therapy or pre-existing conditions/external factors will be important. Again, long-read sequencing, which is more comprehensive and reliable than short-read sequencing, could provide some important answers,” adds ARK.
Data confirm the importance of long-read sequencing to both healthcare companies and investors. There are a massive number of gene therapies currently in clinical trials.
“Recently, ARK estimated the number of active gene therapy clinical trials at 712, 238 of them initiated in 2020. As the shift to gene therapy trials continues, monitoring their safety profiles will be critical,” notes AKRG’s issuer.
Long-read sequencing technology will likely accelerate, too. Despite the massive growth, scientists will do well to continue to observe one principle.
“Gene therapy holds the promise of curing disease and revolutionizing health care. In these early days, however, scientists must focus on its safety,” concludes ARK.
For more on disruptive technologies, visit our Disruptive Technology Channel.
The opinions and forecasts expressed herein are solely those of Tom Lydon, and may not actually come to pass. Information on this site should not be used or construed as an offer to sell, a solicitation of an offer to buy, or a recommendation for any product.

