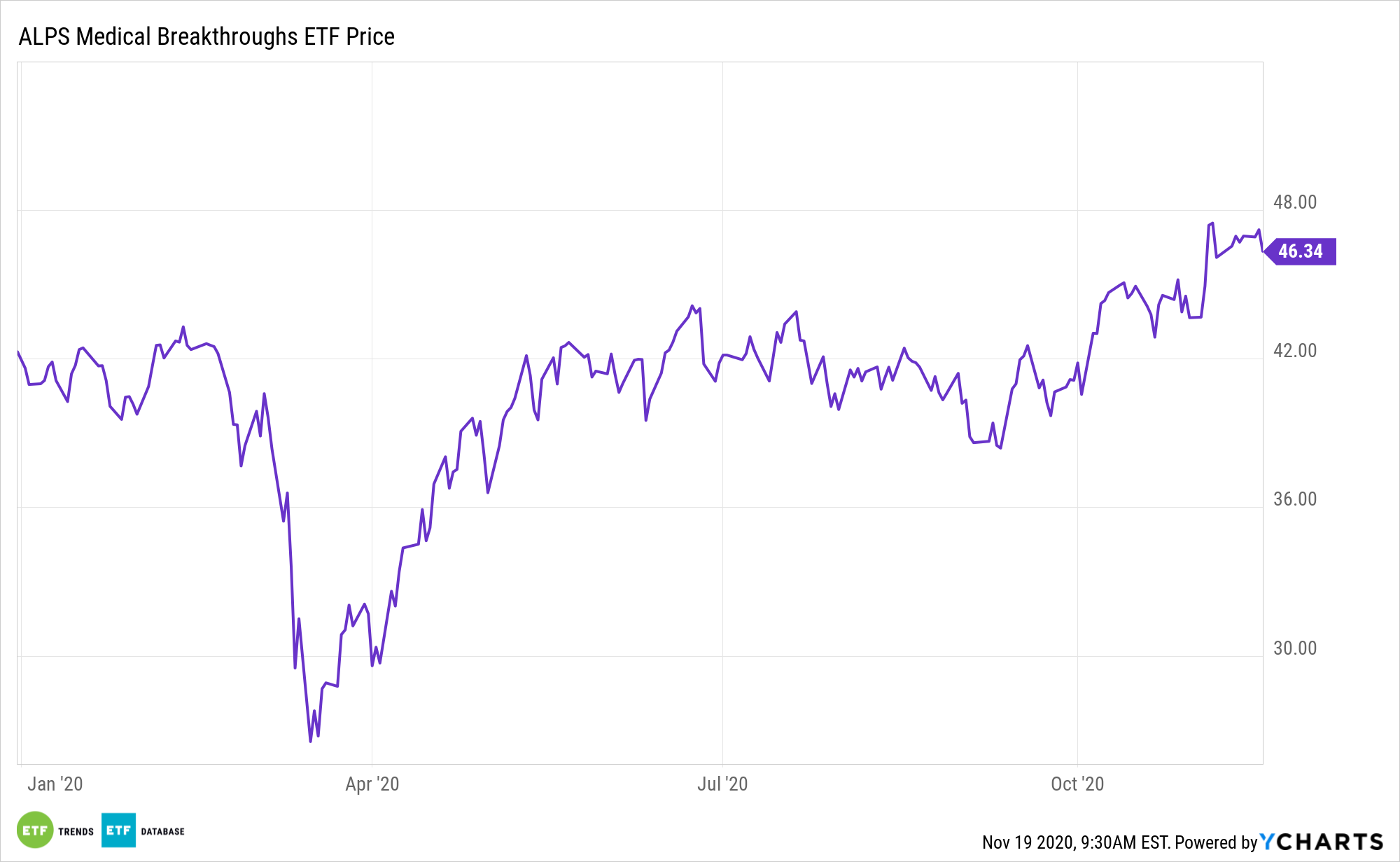
The Food and Drug Administration (FDA) appears likely to be active on the drug review front in the months, with COVID-19 in the way. Expect that activity could benefit exchange traded funds with exposure to small- and mid-cap biotechnology stocks, including the ALPS Medical Breakthroughs ETF (SBIO).
SBIO focuses on small- and mid-cap companies that have one or more drugs in either Phase II or Phase III trials. The component holdings have one or more drugs in either Phase II or Phase III U.S. Food and Drug Administration clinical trials. In a Phase II trial, the drug is administered to a group of 100-300 people to see if it is effective and to evaluate its safety. In a Phase III trial, the drug is given to a larger group, between 500-3,000 people, to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.
“The FDA continues to complete NDA/BLA reviews on time, in light of Covid-19,” write Mizuho analysts. “The FDA has acted on time, and sometimes earlier, on new drug applications with target action (PDUFA) dates in May-October 2020. We believe this is encouraging in light of Covid-19. While companies may be impacted from a commercial and clinical development standpoint to various degrees, there appears to be minimal impact on regulatory review timeline.”
Biotech Investments: Speculative, But Profitable
SBIO’s underlying index, the Poliwogg Medical Breakthroughs Index, is comprised of small- and mid-cap stocks of biotechnology companies that have one or more drugs in either Phase II or Phase III U.S. Food and Drug Administration (FDA) clinical trials.
An example of an SBIO holding that could benefit from a swifter FDA review process is Avrobio Inc. (NASDAQ: AVRO), which is working on treatments for Fabry and Gaucher diseases, among others. Mizuho says in a bull case scenario, that stock could vault to $51, but there are risks to consider.
“Investing in clinical stage companies in the biotech and pharmaceuticals industry is speculative in nature and is only appropriate for those that have high tolerance for price volatility. In addition to the common risks involved with investing in companies in the biotech and pharmaceuticals sectors, such as R&D, manufacturing, regulatory and commercial risks, AVROBIO Inc. has several firm-specific risk,” according to the research firm.
Other biotech ETFs include the VanEck Vectors Biotech ETF (BBH), iShares Nasdaq Biotechnology ETF (IBB), and the Virtus LifeSci Biotech Clinical Trials ETF (BBC).
For more on cornerstone strategies, visit our ETF Building Blocks Channel.
The opinions and forecasts expressed herein are solely those of Tom Lydon, and may not actually come to pass. Information on this site should not be used or construed as an offer to sell, a solicitation of an offer to buy, or a recommendation for any product.