After a lull in mergers and acquisitions activity, Big Pharma could begin to use its cash stores to buy out smaller targets, potentially fueling the momentum in biotechnology-related exchange traded funds that target the small and mid cap segments.
According to Dealogic data, total deal volume for the drug industry reached $253 billion globally in 2020, the Wall Street Journal reports. To put this number in perspective, the markets witnessed a record $432 billion in 2019.
Observers attributed the slowdown in M&A activity to the onset of the coronavirus pandemic, along with election-related uncertainty over future regulatory outlook.
However, investors anticipate things to change in 2021. For starters, the so-called blue wave where both the Oval Office and Congress are both controlled by Democrats did not materialize. Consequently, there are doubts to whether or not broad legislation on regulations would pass through Capitol Hill.
Furthermore, the biotech industry has accumulated some good will after quickly developing a safe and effective Covid-19 vaccine.
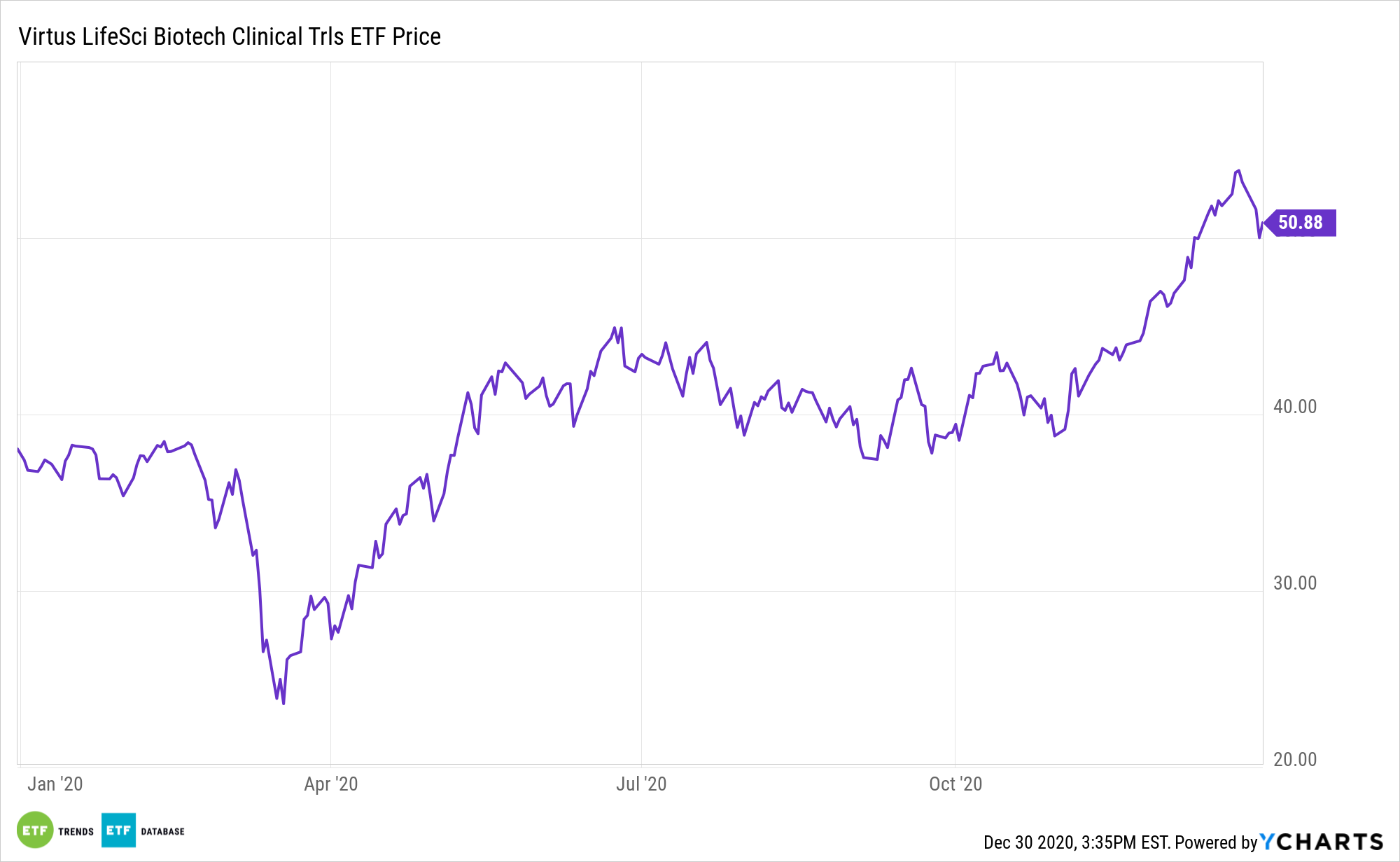
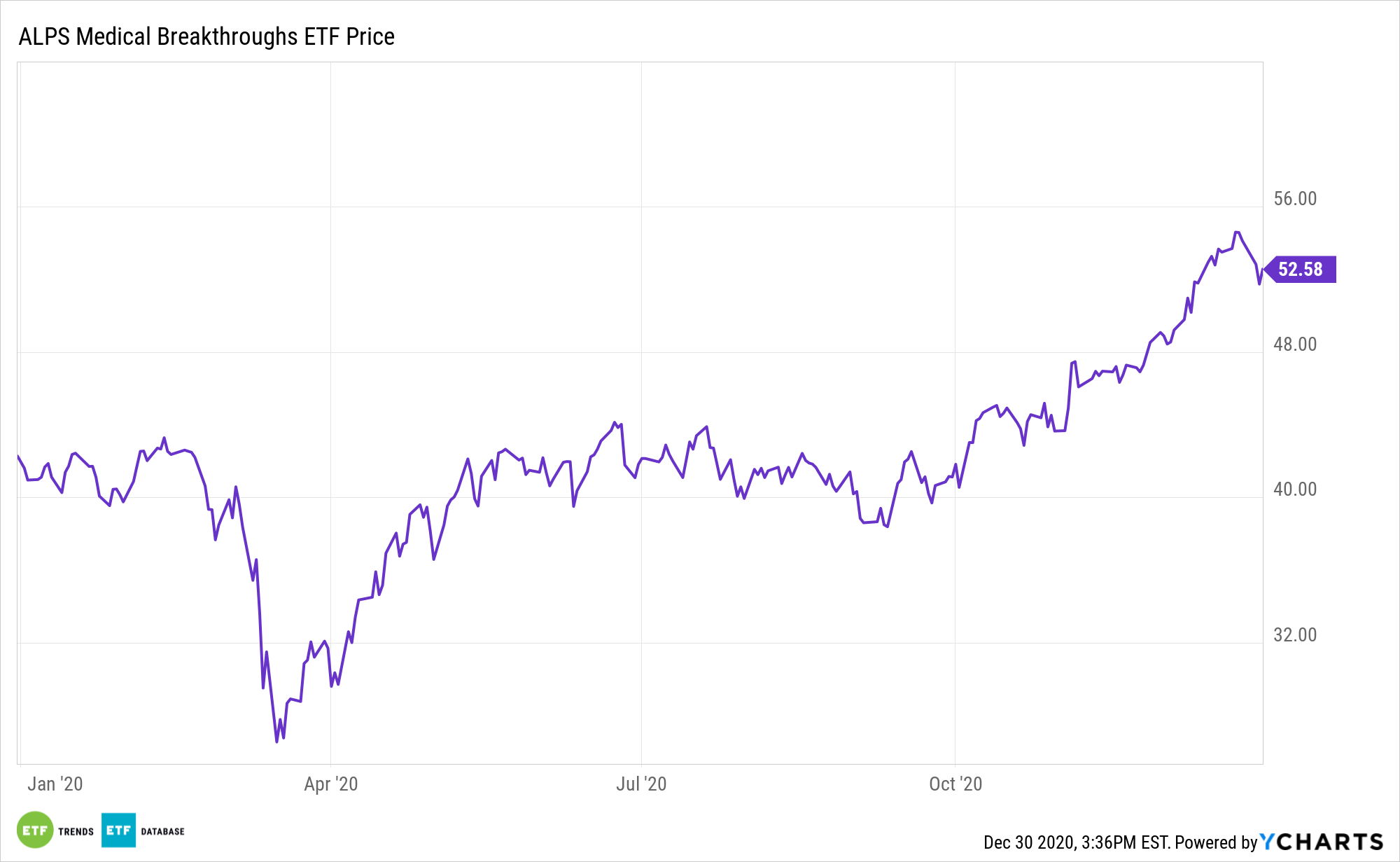
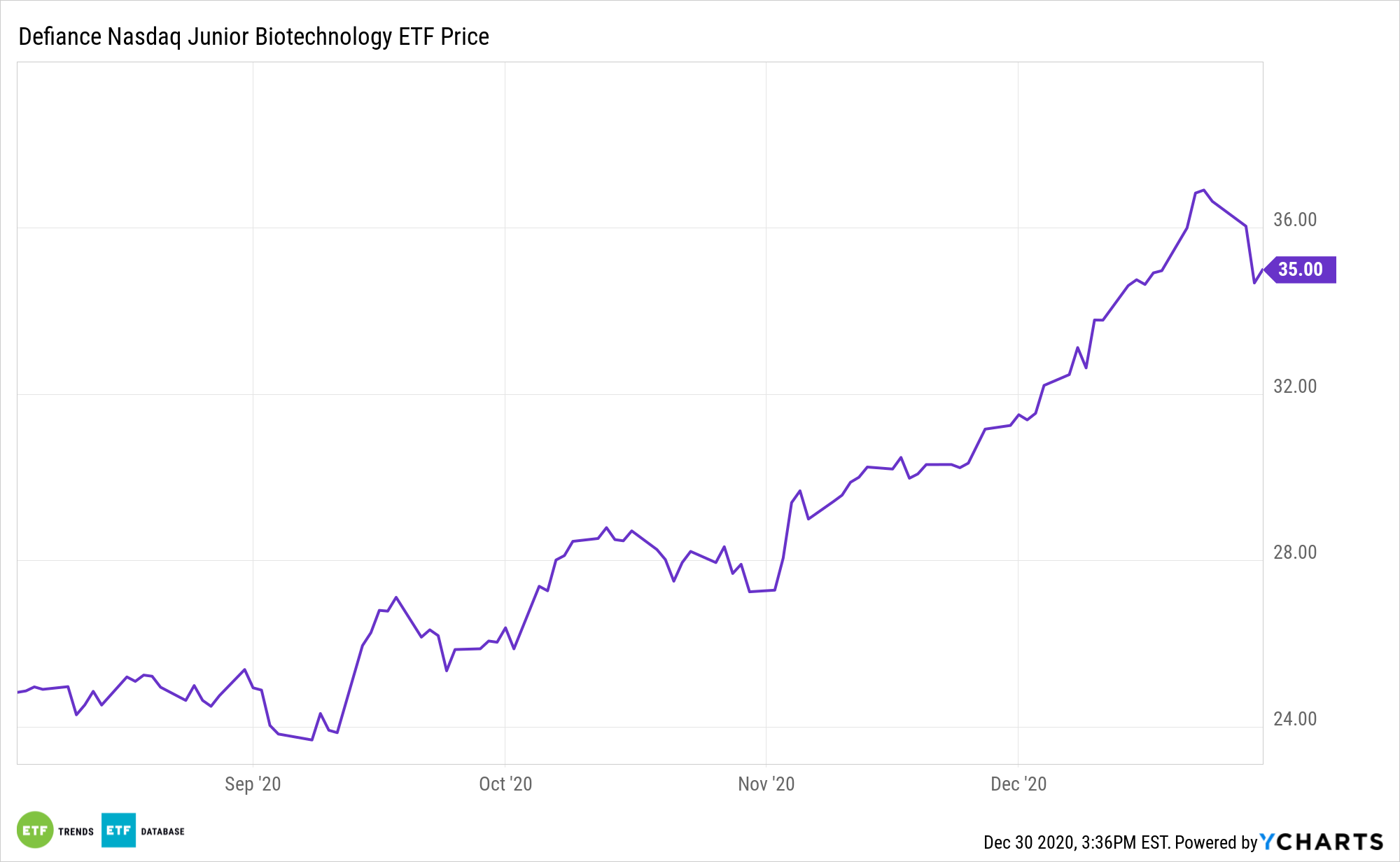
ETF investors who are interested in the potential increased M&A activity in the new year may look to biotech-related ETFs that cover more small- and mid-sized companies that are more attractive targets for Big Pharma. For example, investors can look to the Virtus LifeSci Biotech Clinical Trials ETF (BBC), ALPS Medical Breakthroughs ETF (NYSEArca: SBIO), Defiance Nasdaq Junior Biotechnology ETF (IBBJ), and SPDR S&P Biotech ETF (NYSEArca: XBI).
The Virtus LifeSci Biotech Clinical Trials ETF tracks the BioShares Biotechnology Clinical Trials Index. Clinical Trials stage companies are typically younger, smaller companies which do not have a drug approved, but instead focus on testing their experimental drugs. Companies with a lead drug candidate in Phase 1, Phase 2, or Phase 3 trial can be included in that index. Phase 1 is related to safety and dosage control, where around 70% are generally able to make it through. Phase 2 focuses on side effects and what adverse effects there may be. About a third of those move through to stages beyond, which take into consideration other factors.
The ALPS Medical Breakthroughs ETF focuses on small- and mid-cap companies that have one or more drugs in either Phase II or Phase III trials. The component holdings have one or more drugs in either Phase II or Phase III U.S. Food and Drug Administration clinical trials. In a Phase II trial, the drug is administered to a group of 100-300 people to see if it is effective and to evaluate its safety. In a Phase III trial, the drug is given to a larger group, between 500-3,000 people, to confirm its effectiveness, monitor side effects, compare it to commonly used treatments and collect information that will allow the drug or treatment to be used safely.
The Defiance Nasdaq Junior Biotechnology ETF’s member firms are engaged in biotech research and development, the sale or licensing of biological substances for the purposes of drug discovery and diagnostic development; and pharmaceutical manufacturers of prescription or over-the-counter drugs, including vaccines and development and manufacturing companies.
Lastly, the SPDR S&P Biotech ETF, one of the largest biotechnology ETFs by assets, uses an equal-weight methodology, which means it tilts toward smaller companies, but not at the expense of exposure to some of the industry’s larger players.
For more information on the healthcare industry, visit our healthcare category.