The FDA has officially given full approval to Pfizer and BioNTech for their COVID-19 vaccine, potentially making way for more approvals and more vaccine mandates, reports CNBC. It is a move that officials are hoping will encourage those who have been reluctant to receive a vaccine to get vaccinated.
The federal approval was a first for U.S. vaccine candidates and could give businesses and schools around the country a more firm stance in implementing and enforcing vaccine mandates. Pfizer’s vaccine had previously been approved under an emergency use authorization, along with the Moderna and J&J vaccines.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated,” said acting FDA Commissioner Janet Woodcock in a statement.
FDA scientists analyzed “hundreds of thousands” of pages of vaccine data from over 40,000 trial participants, the FDA said. They concluded that the vaccine is currently 91% effective, as opposed to the 95% efficacy that had been found in trials before Delta. It met the “high standards for safety, effectiveness, and manufacturing quality,” that the FDA upholds, Woodcock said.
“You’re going to see the empowerment of local enterprises, giving mandates that could be colleges, universities, places of business, a whole variety and I strongly support that,” White House chief medical officer Dr. Anthony Fauci said at the beginning of August when speaking on the potential for full approval for a vaccine.
More than 204 million Pfizer-BioNTech shots have been administered across the country, according to CDC data. Trials are still ongoing for children under 12. The vaccine is still in emergency approval for the 12-15 age group as more data is still needed for full approval.
A full approval means the manufacturers can take the vaccine to market once the pandemic is over and the U.S. is no longer under a state of emergency.
Biotech Continues to Thrive in the Midst of Pandemic Variants
As the Delta variant makes its way across the globe, many biotech firms are experiencing a boom from public demand and opportunities unique to the pandemic. The ProShares Ultra Nasdaq Biotechnology ETF (BIB) is a leveraged option for investors looking for exposure to biotechnology and pharmaceuticals during bullish times for the industry.
The fund captures twice the daily return of the underlying Nasdaq Biotechnology Index before fees and taxes. The exposure resets daily, and as such, does not provide a simple 2x multiplier on the return of the underlying index.
Accordingly, BIB should be monitored regularly by potential investors.
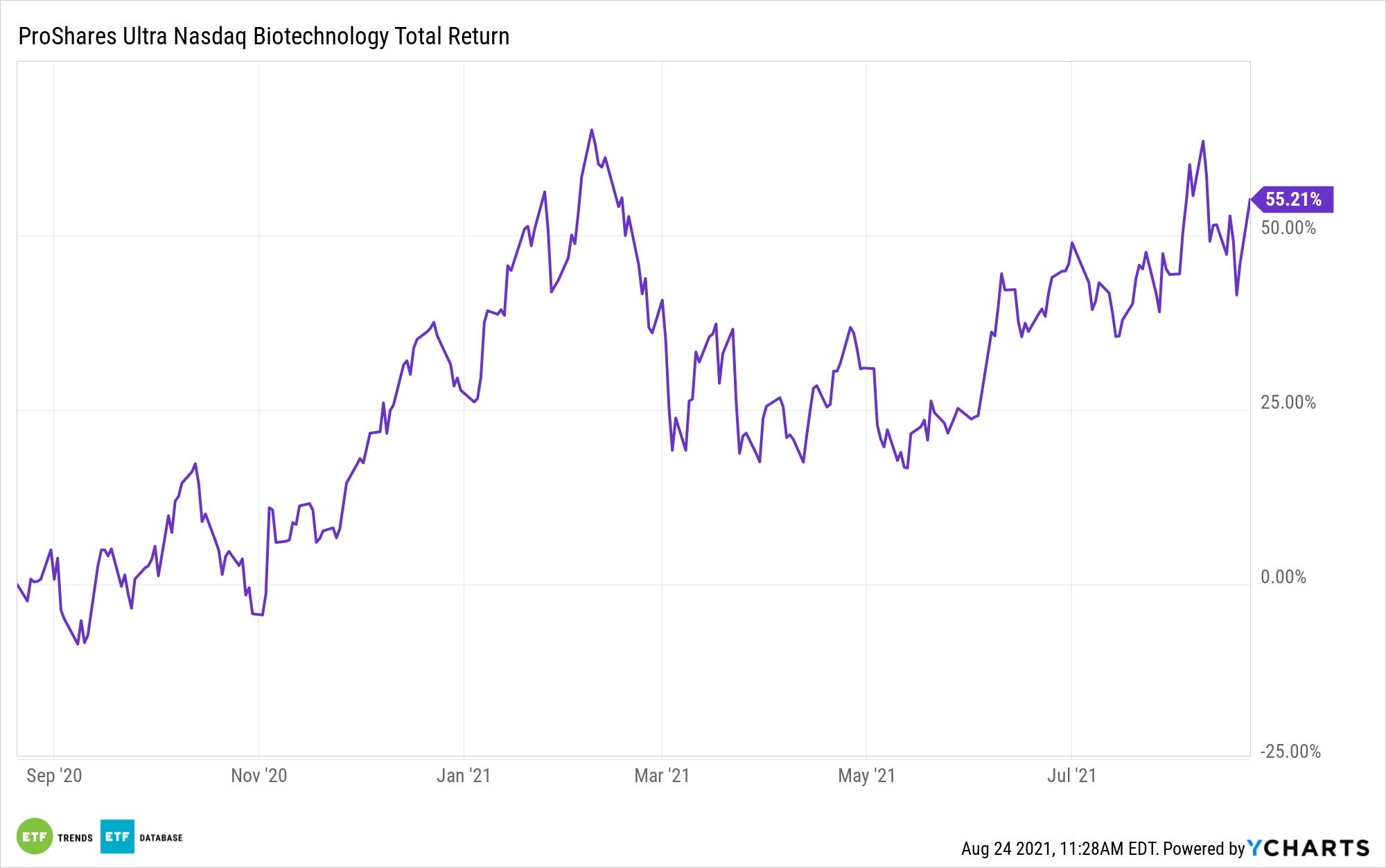
By utilizing swap agreements with major banking institutions and investing in shares of the companies tracked within the Index, such as BioNTech (BNTX) and Moderna (MRNA), BIB rebalances daily to align with the Index.
BIB carries an expense ratio of 0.95%, with a contractual waiver that ends 9/30/21.
For more news, information, and strategy, visit the Nasdaq Portfolio Solutions Channel.

